ANTI-CORROSIVE, ANTI ALLERGIC,
ANTI-THROMBOGENIC,
SIMPLY PERFECT.
SEQUENCE™
The combination of Sirolimus antiproliferative action combined with biodegradable polymer and a proven balloon catheter sets new standards in treatment of clinically complex lesions.
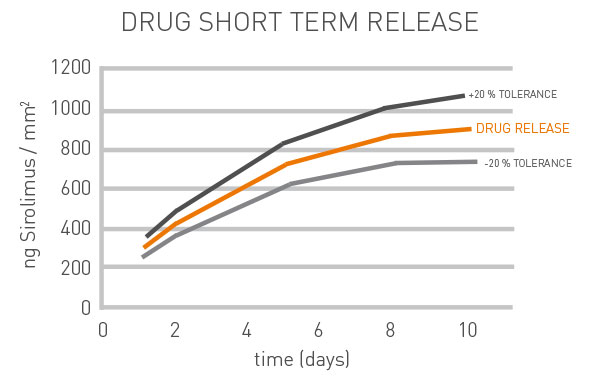
Carrier coating and drug are absorbed simultaneously within three months, which covers exactly the time where restenosis tend to occur most frequently.
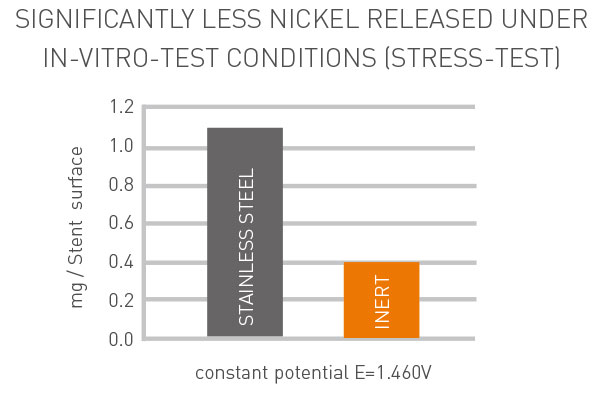
Only a stent platform treated with inert carbon technology is left behind preventing any leakage of metals and avoiding allergic immune response reactions to stent implantation.
Sirolimus-eluting
Completely bioabsorbable within 3 months
No auxiliary polymer used
Reliable and even drug release
No leakage of heavy metal ions
Inhibition of thrombin synthesis
Low inflation pressures
Uniform stent expansion
Optimal hydrophilic coating for maximized crossability
High radial strength combined with high flexibility due to Open Cell design
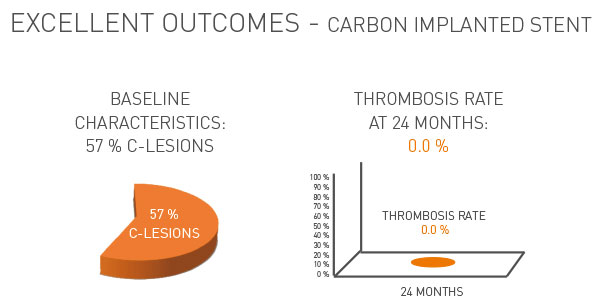
Carbon implanted stent surface for proven higher biocompatibility and highly reduced restenosis rate
TECHNICAL SPECIFICATIONS

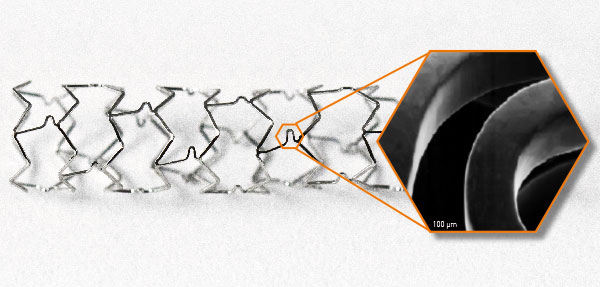
ADVANCED INERT CARBON TECHNOLOGY
The innovation of this stent lies in the advanced inert carbon technology. Under vacuum carbon particles are shot with high load of energy in the metal structure, so that they are implanted within the metal lattice under the alloy´s surface.
COATING
- PLGA polymer – uniform, localized drug delivery
- Polymer biodegradation period: 6 weeks
- Polymer thickness: approx. 5 µm
- Drug: Sirolimus (Rapamycin)
- Drug loading: 2.0 µm/mm2
- Coating degrades 100% into carbon dioxide and water
- Eliminating inflammation, stent thrombosis and MACE
- Suppresses neointimal growth without suppressing endothelialization
- Simultaneous controlled polymer degradation, prevents restenosis in most critical period
- Quick arterial wall healing, restores vessel physiology
- Significantly reduces C-lesions through ion implanted stent platform technology
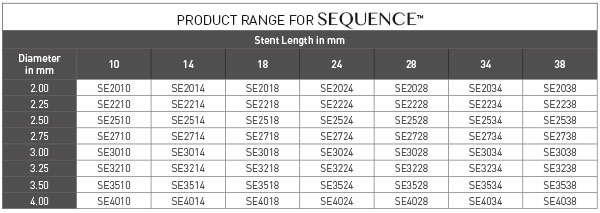
PRODUCT ORDERING INFORMATION