THE ONE AND ONLY COMPLETELY
BIODEGRADABLE PERIPHERAL
STENT IN THE WORLD
REMEDY™
To date REMEDY™ is the only CE certified biodegradable peripheral stent in the world.
Restenosis, thrombosis and Immune response reactions caused by in place stents are prevented and duration of antiplatlet therapy reduced.
EXCLUSIVELY
Through a special partnership between KMP & ENDOCOR we can offer you this unique stent exclusively.

Flexibility
Stent material
Applications
DELIVERY SYSTEM INFORMATION
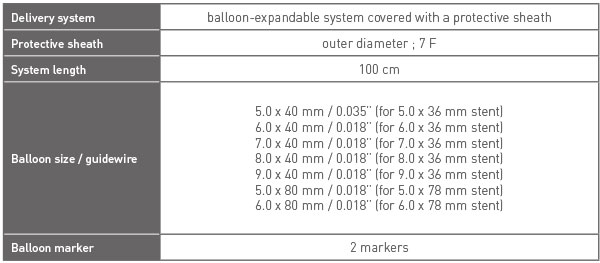
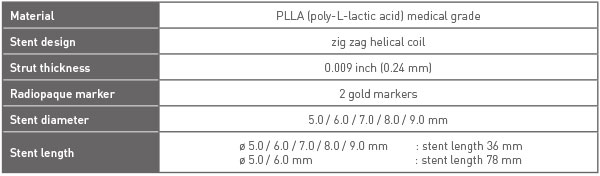
STENT INFORMATION
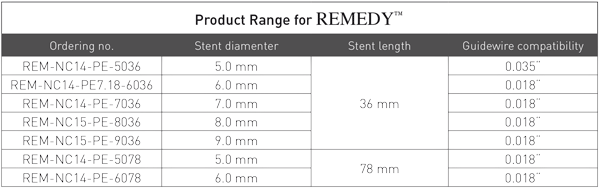
PRODUCT ORDERING INFORMATION
Test
| Ordering No. | Stent diameter | Stent lenght | Guidewire |
|---|---|---|---|
| REM NO | 5mm | 36mm | 0,01312 |
| REM NO | 5mm | 36mm | 0,01312 |
| REM NO | 5mm | 36mm | 0,01312 |
| REM NO | 5mm | 36mm | 0,01312 |
| REM NO | 5mm | 36mm | 0,01312 |
| REM NO | 5mm | 36mm | 0,01312 |
ALSTER STUDY, SPECTRUM STUDY
ENDOCOR has launched the ALSTER study – a multi centre global study running in several centers all over the world enrolling as much as 600 patients to evaluate the safety and efficacy of the REMEDY – Completely Biodegradable Peripheral Stent for the treatment of SFA and Iliac lesions.
Parallel to the ALSTER STUDY Endocor is also running the SPECTRUM study – a smaller study for the REMEDY stent in selected centres in Germany and Austria enrolling 300 patients.
The objective of this study is to provide more data to support the only CE certified biodegradable peripheral stent in the world which allows for a temporary implant designed to perform during period of high risk and to disappear later from the body completely.
We believe in the future of stents with biodegradable materials which will make interventions safer on a long term perspective, avoid negative effects of an inbody implant and contribute to patients’ health and successful treatment.



